-
 United European Gastroenterology Week 2018
United European Gastroenterology Week 2018United European Gastroenterology Week 2018 ● Date: Oct 22nd to 24th, 2018● Venue: Austria Center Vienna, Vienna, Austria● Booth Exhibition & Taewoong Medical Dinner Symposium The 26th United European Gastroenterology Week (UEGW) was held at Austria Center Vienna from October 22nd to the 24th. As always, Taewoong Medical participated as an exhibitor and enjoyed lively interactions between industries and doctors. The newly released products including the Biliary S Flare stent and the Esophageal Dual stent were exposed to the public for the first time and achieved huge attention from the attendees. In addition, the EUSRA RFA device which was approved by the US FDA right before the congress also drew extensive interest.To measure up the high interest in the advanced EUS-guided procedure, Taewoong Medical prepared a Dinner symposium under the theme of "EUS-guided stenting and radio frequency ablation." For this special event, four prominent experts were invited and they shared their extracted know-hows to the guests.
18.11.05 -
 SNUH’s artificial heart valve wins regulators’ approval
SNUH’s artificial heart valve wins regulators’ approvalSNUH’s artificial heart valve wins regulators’ approval Seoul National University Hospital (SNUH) said Thursday that it has received sales approval from the Ministry of Food and Drug Safety for its pulmonary artery artificial heart valve. The hospital has been researching the device along with TaeWoong Medical since 2014.The research team, led by Professors Kim Ki-bum, Kim Yong-jin and Im Heung-gook at the hospital, began the development of artificial heart valves using pig and bovine adrenal glands with the support from the long-term biosimilar project team funded by the Ministry of Health and Welfare. The team has also developed a stent that does not require patients to undergo an open chest surgery.After completing animal research, it conducted a clinical trial by transplanting the artificial heart valve on 10 patients and followed up on them for six months to prove the device’s effectiveness and safety in 2016.As a result, the patient did not require any immunosuppressant as the valve showed almost no immune rejection, which is the biggest problem of xenotransplantation. Professor Kim Ki-bum “Currently, there are inquiries about the commercialization of the product not only from Asian countries such as Japan, Taiwan, and Hong Kong but Europe,” the hospital said. “The research team will meet with 11 pediatric cardiac centers in six European countries next month and will begin clinical trials early next year.”The heart has four valves that control blood circulation. The most common valve disease is valve stenosis of the aorta. However, TAVI, an aorta artificial heart valve, developed by advanced countries such as the U.S. has already secured most of the market share.The hospital expects to have similar benefits, as the pulmonary artery artificial heart valve developed by SNUH research team is the first commercialized product of its kind in the world. Until now, Korea, the United States and China have been fiercely competing to develop the product.The valve and stent developed by SNUH do not require open chest surgery and has a firm stent that can match the size of the patient’s pulmonary artery. The hospital expects that such benefits can lower the financial burden on patients.”Currently, additional clinical trials are underway at several hospitals in Korea,” Professor Kim Ki-bum said. “If the hospital manages to receive a European CE certification after clinical trials in Europe, it will contribute to the improvement of patient's quality of life and globalization of Korean medical technology.”corea022@docdocdoc.co.kr<© Korea Biomedical Review, All rights reserved.>http://www.koreabiomed.com/news/articleView.html?idxno=4414
18.11.05 -
 CONNECT GLOBAL issue 9, 2018 18.11.05
CONNECT GLOBAL issue 9, 2018 18.11.05 -
 Digestive Disease Week 2018
Digestive Disease Week 2018Digestive Disease Week 2018 ● From: June 2nd to June 5th, 2018● Location: Walter E. Washington Convention Center, Washington DC● Booth Exhibition & Taewoong Medical’s Dinner Party Breaking News that will change the course of the US Market and open a New Era. The Digestive Disease Week® (DDW) 2018 annual meeting took place in Washington, D.C., from June 2nd to the 5th. Taewoong Medical, as we have been doing during the past 12 consecutive years, participated in the largest Gastrointestinal Medical event in USA as an exhibitor with great honor.Lumen-Apposing Metallic Stents (LAMS) such as Taewoong’s own SPAXUS™ and NAGI™ stents, and Endoscopic Radio Frequency Ablation (Endoscopic RFA) devices such as EUSRA™ and ELRA™ received extensive attention from the congress attendees throughout the entire event this year. The fever of this year’s interest and joy created through the DDW event lasted not only during the exhibition hours, but also all night through the evening as Taewoong enjoyed an impressive evening with its partners and customers from all over the world at the Nationals Park, Washington DC’s MLB (Major League Baseball) Stadium. Last but not least, the announcement of Taewoong and Cook’s partnership in the US market gained a lot of attention from the event attendees as it was the start of a new era that will bring great benefits and development to not only both partners but also to the customers and patients in all. It was a great opportunity to see and feel firsthand the changing trends of the new market at such an enormous medical event and a great pleasure to meet and respond directly to our customers from around the world.
18.06.11 -
 Cook Medical, Taewoong Medical Partner to Distribute Stents in the U.S.
Cook Medical, Taewoong Medical Partner to Distribute Stents in the U.S.Cook Medical, Taewoong Medical Partner to Distribute Stents in the U.S. BLOOMINGTON, Ind.–(BUSINESS WIRE)–Jun 4, 2018–To complement its existing line of metal GI stents, Cook Medical has partnered with Taewoong Medical to distribute a selection of stents into the U.S. The new partnership includes the Niti-S™ Through the Scope (TTS) Esophageal Stent, the flagship product of the South Korean company’s Niti-S Self-Expandable line of metal GI stents. Cook Medical will begin distributing the Taewoong portfolio on November 1, 2018.Esophageal stents are often used in the treatment of esophageal cancer. According to the American Cancer Institute for Cancer Research, there are approximately 17,000 new cases reported annually, the vast majority occurring in men. This year alone, esophageal cancer will claim almost 16,000 lives. “We’re excited to continue our goal to support physicians in their treatment of diseases of the GI tract by adding Taewoong’s stent technologies to our existing Evolution® and Zilver® stent offerings,” said Barry Slowey, president of Cook Winston-Salem and vice president of Cook Medical’s Endoscopy speciality. “In addition to their product offering, we’re honored to collaborate with Taewoong because we share a commitment to provide solutions that deliver patient care through innovation.”Unique to the U.S. market, the Niti-S TTS Esophageal Stent is preloaded in a 10.5 Fr delivery system. This allows deployment through a scope with a conical tip designed to navigate tight strictures and tortuous anatomies.In addition to the Niti-S TTS Esophageal Stent, Cook will also be the U.S. distributor for the Niti-S S Esophageal Stent—a standard esophageal stent—as well as the Niti-S Biliary Stent.“We are very pleased and proud to enter into this partnership with Cook Medical, a leading global medical device company,” said Kyongmin Shin, CEO of Taewoong Medical. “We believe Cook Medical’s philosophy and focus towards continuous innovation in product development is akin to Taewoong Medical’s own philosophy. And I am confident that our partnership will bring the optimal stent solutions to U.S. doctors and patients.”About Cook MedicalSince 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.About Taewoong MedicalTaewoong Medical Co., Ltd, founded in 1991, is a privately-held company based in Seoul, South Korea. It manufactures a range of medical equipment of its own design: gastrointestinal devices, interventional urology stents and GI accessories. Taewoong is committed to maintaining high levels of innovation, knowledge and technical expertise to deliver solutions to their customers.
18.06.06 -
 United European Gastroenterology Week 2017
United European Gastroenterology Week 2017United European Gastroenterology Week 2017 ● Oct.30 ~ Nov.02, 2017● Congress Venue: Fira Barcelona Gran Via, Barcelona, Spain● Booth Exhibition & Taewoong Medical’s Dinner Symposium The 25th UEGW has ended successfully. This year it was held in Gaudi’s hometown of Barcelona, Spain. Taewoong Medical participated in the exhibition with mainly Gastrointestinal Metal stent and Endoscopic RFA products as well. In particular, this year, the same stent companies participated in 12 venues, and it was able to see how stent competition is getting stronger every each year.Even in the midst of intense competition, Taewoong Medical always strives to show that quality products are always improving. As time goes on, we could feel the growing interest in RFA products. Currently, RFA products are undergoing study in many countries around the world. We are looking forward to seeing you in more countries soon. Many people attended to Taewoong Medical dinner symposium, which is celebrating its 7th anniversary this year. This year, a lecture on “ERCP guided radiofrequency ablation” and “Transmural fenestrations: how to keep the window open?” was held. In addition to the lecture, we prepared special time for who attended our dinner symposium. The Dinner symposium was completed with the participation of the enthusiastic flamenco performances of Spain. Next UEGW will be held at Vienna, Austria from Oct.20th to Oct.24th 2018. We are looking forward to seeing you again next year.
17.11.13 -
 CONNECT GLOBAL issue 8, 2017 17.11.07
CONNECT GLOBAL issue 8, 2017 17.11.07 -
 Digestive Disease Week 2017
Digestive Disease Week 2017Digestive Disease Week 2017 ● May 7~9, 2017● Congress Venue: Mccormick Place, Chicago, U.S.A● Booth Exhibition & Taewoong Medical’s Dinner Party We took part in DDW 2017 which was taken place in Mccormick Place, Chicago, U.S.A. The exhibition booth consisted of not only Stent, Endoscopic RFA, SPAXUS area but multi-entrance, meeting room, sofas and table with phone recharger for visitor’s convenience. Modern and simple design, emphasizing SPAXUS and RFA, caught lots of attention. Second day of DDW, about 180 Doctors and distributors from many countries enjoyed dinner with tremendous view at Taewoong Medical’s Niti-S Cruise Dinner, just like 3 years ago. 4 the Beat, invited band, played music during dinner followed by dance party with the DJ. Next DDW will be held at Washington Convention Center from June 2nd to June 5th 2018. We always welcome you to join our program.
17.05.23 -
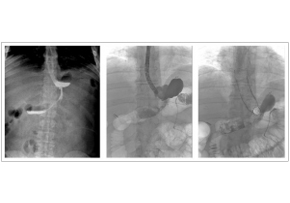 Integrated Approaches for the Management of Staple Line Leaks following Sleeve Gastrectomy
Integrated Approaches for the Management of Staple Line Leaks following Sleeve GastrectomyJ Obes. 2017; 2017: 4703236. Integrated Approaches for the Management of Staple Line Leaks following Sleeve Gastrectomy Endoscopic stenting after staple line leaks has been supported by many authors in recent years, even if this is not a widely accepted treatment. Stent migration is the main complication after the procedure and it has been reported in 20%–59% of cases among different series. In this experience, stents 20 cm long and 24 mm in diameter BETA™ Stents were chosen. In this way, in each case, a good adherence of the stent to the gastric wall. Male, 34 years, BMI 43 Kg/m2. Evidence of leak in radiological upper gastrointestinal series; confirmation of the leak with fluorescence endoscopy; endoscopic gastrooesophageal stenting.
17.04.10 -
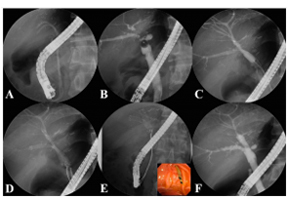 Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after liv
Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after livTherap Adv Gastroenterol. 2017 Mar; 10(3): 297–309. Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after living-donor liver transplantation Consequently, the aim of this study was to examine the feasibility and efficacy of using a newly designed fully covered self-expandable metal stent (FCSEMS) to resolve refractory ABS. A total of 35 FCSEMSs were positioned endoscopically and removed after 2-3 months. The anastomotic stricture resolved with the FCSEMS insertion in 29 of 35 patients (clinical success rate: 82.9%). The newly designed FCSEMS: KAFFES™ Stent is a potentially feasible and effective treatment for anastomotic strictures that develop after LT but are not amenable to treatment by conventional procedures. Insertion procedure for multiple FCSEMSs. (A) Previously inserted plastic stents are removed endoscopically. (B) The cholangiogram shows multiple anastomotic strictures at the posterior and inferior intrahepatic ducts. (C) The strictures are dilated using a balloon dilator to allow passage of the FCSEMSs. (D) The FCSEMSs are inserted sequentially into the stricture sites. (E) After an indwelling time of 2–3 months, the FCSEMSs are removed by grasping the retrieval strings using biopsy forceps. (F) The cholangiogram demonstrates resolution of the multiple strictures.
17.04.10 -
 Hands-on training of ENDO 2017 (Hyderabad, India)
Hands-on training of ENDO 2017 (Hyderabad, India)Hands-on training of ENDO 2017 (Hyderabad, India) Taewoong Medical supported a hands-on training program by KSGE (Korea Society of Gastrointestinal Endoscopy) and TAGE (Thai Association of Gastrointestinal Endoscopy) at the ENDO 2017 (16th-19th Feb) conference in Hyderabad, India. KSGE Hands-on Training (Topic: Metal stenting)KSGE hands-on training provided to give the over 40 participants chance to actually insert and deploy the plastic and/or metal stent endoscopically in the dummy model. This training program consisted of 6 stations including esophagus and/or pylorus stenting, colon stenting, bile duct plastic stenting, CBD metal stenting and hilar bile duct stenting (stent-in-stent and stent-by-stent). Honorable ERCP and/or Endoscopy masters, KSGE members offered one-on-one coaching and shared their valuable clinical skills and knowledge. The participants could have enhanced their skills on basic cannulation, stricture management with self-expandable metal stents. TAGE Hands-on Training (Topic: EUS-guided drainage)At this program, expert tutoring was available at one station for fine needle aspiration cytology and biopsy, two stations for EUS biliary drainage with metal stent and three stations for EUS pseudocyst drainage with plastic stent and NAGI™ and SPAXUS™ stent. Participants had a proctoring opportunity to observe and experience advanced therapeutic EUS techniques.
17.03.02 -
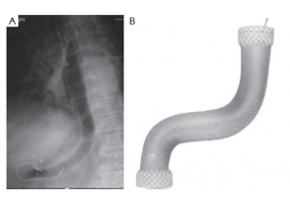 A novel management of post-oesophagectomy gastro-pleural fistula
A novel management of post-oesophagectomy gastro-pleural fistulaJ Gastrointest Oncol 2016;7(6):E93-E97 A novel management of post-oesophagectomy gastro-pleural fistula Javaid Ishtiaq1, Jonathan Sutton2, Waqar Ahmed2 AbstractOesophageal anastomotic leak and fistula are major and life-threatening complications of oesophagectomy with resultant increased mortality. Non-operative approach of such cases should be the initial strategy. Re-operative surgery and/or stent insertion are considered if conservative measures failed. Although oesophageal stenting is a safe option for the leaks, stent migration and failure to completely cover large anastomotic leaks are the main complications and pitfalls of the procedure. These can be overcome by using multiple or larger stents. We describe a case of a 73-year-old man who underwent a laparoscopic oesophagectomy for an oesophageal adenocarcinoma. The procedure was complicated by a large gastro-pleural fistula and anastomotic leak, resulting into a chronic empyema. The initial conservative treatment and a conventional oesophageal stent insertion failed to heal the fistula and to resolve the empyema. Re-operative surgery was ruled out because of the patient’s poor general health and high surgical risk. Due to the changed oesophago-gastric anatomy and a potential risk of migration of the additional conventional stent, a mega stent was deployed with successful closure of the oesophageal leak. Post-stenting contrast studies and an out-patient follow up review of the case confirmed no further anastomotic leakage.KeywordsGastrointestinal fistula; oesophageal leak; oesophageal stent; oesophageal cancer; mega stent Figure 3. The large (Mega) stent covering the oesophagus, stomach and pylorus with its proximal end in the previously deployed oesophageal stent and the distal end in the duodenum (A). The Mega stent (Taewoong Medical Niti-S Gyeonggi-do, South Korea oesophageal covered stent) (B). Correspondence to: Dr. Javaid Ishtiaq. Specialty Registrar, Gastroenterology, Sandwell and West Birmingham Hospitals NHS Trust, Lyndon, West Bromwich, West Midlands, B71 4HJ, UK. Email: javaidishtiaq@yahoo.com.
17.02.28 -
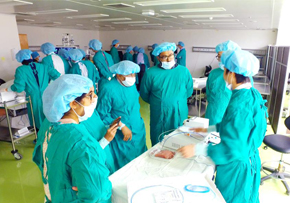 2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program)
2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program)2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program) 2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program) was held in Seoul, Korea from 6th to 7th January. Like last 1st AERAT training, many doctors participated in this time. A total of 12 doctors from HongKong, Malaysia, Singapore, India and Japan attedned and experienced various EUS related programs for 1night 2days. AERAT’s director Prof. Dong Wan, Seo gave a lecture related with EUS-procedure cases and his valuable experiences with attendees. Also, we prepared hands on session at Asan Meical Center Animal Laboratory with Phatom model. ex-vivo and procine model tests as well. All of attendees had their own experiences with our EUSRA probe. All the participants gave a good feedback that AERAT program was a satisfactory training course. We hope to meet many doctors in our RFA traiing class near in the future.
17.01.20 -
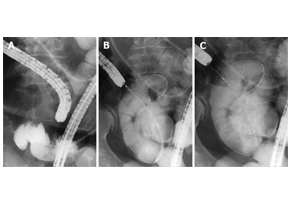 Niti-S™ Colon D Stent_malignant small bowel obstruction
Niti-S™ Colon D Stent_malignant small bowel obstructionWorld J Gastroenterol. 2016 Oct 28; 22(40): 9022–9027. Safety and efficacy of self-expandable metallic stents in malignant small bowel obstructions Akiyoshi Tsuboi, Toshio Kuwai, Tomoyuki Nishimura, Sumio Iio, Takeshi Mori, Hiroki Imagawa, Toshiki Yamaguchi, Atsushi Yamaguchi, Hirotaka Kouno, Hiroshi Kohno AbstractIn this report, we present 3 cases of malignant small bowel obstruction, treated with palliative care using endoscopic self-expandable metallic stent (SEMS) placement, with the aim to identify the safety and efficacy of this procedure. Baseline patient characteristics, procedure methods, procedure time, technical and clinical success rates, complications, and patient outcomes were obtained. All 3 patients had pancreatic cancer with small bowel strictures. One patient received the SEMS using colonoscopy, while the other 2 patients received SEMS placement via double balloon endoscopy using the through-the-overtube technique. The median procedure time was 104 min. The technical and clinical success rates were 100%. Post-treatment, obstructive symptoms in all patients improved, and a low-residue diet could be tolerated. All stents remained within the patients until their deaths. The median overall survival time (stent patency time) was 76 d. SEMS placement is safe and effective as a palliative treatment for malignant small bowel obstruction.KeywordsSelf-expandable metallic stents; Malignant small bowel obstructions; Endoscopy; Case report; Pancreatic cancer Figure 1 Self-expandable metallic stent deployment using the standard through-the-scope technique under fluoroscopic guidance. A: The scope was advanced to the stricture, and a standard guidewire was passed through the stricture; B: The stent delivery system was advanced through the scope across the stricture; C: The stent can be seen successfully deployed across the stricture.
17.01.04 -
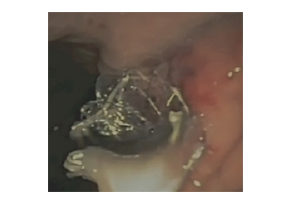 Niti-S™ NAGI™ Stent_A two-center Comparative study of plastic and FCSEMS stent in PFCs
Niti-S™ NAGI™ Stent_A two-center Comparative study of plastic and FCSEMS stent in PFCsEndosc Ultrasound. 2016 Sep-Oct; 5(5): 320–327. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections Tiing Leong Ang, Pradermchai Kongkam,1 Andrew Boon Eu Kwek, Piyachai Orkoonsawat,1 Rungsun Rerknimitr,1 and Kwong Ming Fock Background and ObjectivesEndoscopic ultrasound-guided drainage of walled-off pancreatic fluid collections (PFCs) (pseudocyst [PC]; walled-off necrosis [WON]) utilizes double pigtail plastic stents (PS) and the newer large diameter fully covered self-expandable stents (FCSEMS) customized for PFC drainage. This study examined the impact of type of stent on clinical outcomes and costs.Patients and MethodsRetrospective two-center study. Outcome variables were technical and clinical success, need for repeat procedures, need for direct endoscopic necrosectomy (DEN), and procedure-related costs.ResultsA total of 49 (PC: 31, WON: 18) patients were analyzed. Initially, PS was used in 37 and FCSEMS in 12. Repeat transmural drainage was required in 14 (PS: 13 [9 treated with PS, 4 treated with FCSEMS]; FCSEMS: 1 [treated with PS]) due to stent migration (PS: 3; FCSEMS: 1) or inadequate drainage (PS: 10). Technical success was 100%. Initial clinical success was 64.9% (25/38) for PS versus 91.7% (11/12) for FCSEMS (P = 0.074). With repeat transmural stenting, final clinical success was achieved in 94.6% and 100%, respectively (P = 0.411). Compared to FCSEMS, PS was associated with greater need for repeat drainage (34.2% vs. 6.3%, P = 0.032). The need for and frequency of DEN was similar between both groups, but PS required more frequent balloon dilatation. PS was significantly cheaper for noninfected PC. Costs were similar for infected PC and WON.ConclusionPS was associated with a higher need for a second drainage procedure to achieve clinical success. The use of FCSEMS did not increase procedural costs for infected PC and WON.KeywordsEndosonography, endotherapy, necrosectomy, pancreatic necrosis, pseudocyst Figure 3. Endoscopic image of Nagi™ stent inserted for drainage of infected walled-off necrosis
17.01.03 -
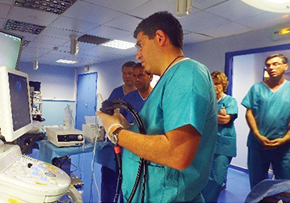 EUS-guided RFA Master Course Program (Seoul, Marseille)
EUS-guided RFA Master Course Program (Seoul, Marseille)EUS-guided procedures are no longer the future of EUS but rather the present! Nowadays EUS has emerged as an essential field in diagnostic and therapeutic endoscopy. Our EUS-guided RFA master course is not only to provide the opportunities to understand mechanism of EUS-guided RFA, but also to learn the essential technical tips for successful RFA applications through comprehensive lectures and hands-on courses including patient’s case observation and animal test, phantom hands-on. We are convinced that our comprehensive and intensive 2 days course will guide all of attendees to EUS-guided RFA program practice as well as introduce our EUS-RFA novel device. We are completed twice of EUS-guided RFA master course successfully until now in Seoul, Korea and Marseille, France.Let’s share the program inside story together from now.EUS-guided RFA Master Course Program is primarily focused on the following training courses and lectures. First program was held in Seoul, Korea Asan Medical Center directed by Prof. Seo, Dong Wan and second program was held in Marseille, France Nord Hospital directed by Prof. Marc Barthet. Both training program were consisted of EUS lectures by director and EUS-RFA case observation, and hands-on with phantom model and porcine model. EUS lectures are open to questions from the participant’s review of case observation and hands-on training in a preliminary session.This one-to-one interaction training program will hopefully build up links and long term relationships helpful for personal development and a greatest integration of future procedure.Most of the participants hope that EUSRA™ can be applied to a variety of indication not only pancreas tumor but also, gradually predict increase these EUS treatment in the future.
16.12.05
+82 31-904-6196contact@stent.net
HOME
NEWS
News
